orbital notation for nickel|electron orbital notation worksheet : iloilo The total number of electrons in nickelis twenty-eight. These electrons are arranged according to specific rules in different . Tingnan ang higit pa The Escape Hunt Experience: Wow!! - See 25 traveler reviews, 6 candid photos, and great deals for Makati, Philippines, at Tripadvisor.
PH0 · orbital notation chart
PH1 · orbital notation calculator
PH2 · orbital diagram for nickel
PH3 · orbital diagram for all elements
PH4 · noble gas notation for nickel
PH5 · lewis dot diagram nickel
PH6 · electron orbital notation worksheet
PH7 · construct the orbital diagram for nickel
PH8 · Iba pa
Ang liham ay isang pahayag o mensahe sa pamamagitan ng pagsulat mula sa isang tao patungo sa isa pang tao o grupo, kadalasan sa ibang lugar.. Bahagi ng Liham 1.Ulong sulat -dito makikita ang pangalan,impormasyon, at lokasyon. 2.Petsa -kung kailan ito sinulat. 3.Patunguhan -nakalagay dito kung saan nais iparating ang liham.
orbital notation for nickel*******The electron configuration of nickel shows that the last shell of nickel has two electrons and the d-orbital has a total of eight electrons. Therefore, the valence electrons of nickelare ten. There are two types of nickel ions. The nickel atom exhibits Ni2+ and Ni3+ ions. The nickel atom donates . Tingnan ang higit paThe total number of electrons in nickelis twenty-eight. These electrons are arranged according to specific rules in different . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paorbital notation for nickelAtoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of nickel is 1s2 2s2 2p6 3s2 3p6 3d8 4s2. In the nickel ground-state electron configuration, the eight electrons . Tingnan ang higit paorbital notation for nickel electron orbital notation worksheetAtoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of nickel is 1s2 2s2 2p6 3s2 3p6 3d8 4s2. In the nickel ground-state electron configuration, the eight electrons . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum . Tingnan ang higit pa
That is, the orbital notation of nickel is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when .
To write the orbital diagram for the Nickel (Ni) first we need to write the electron configuration for just Ni. To do that we need to find the number of ele.
In the case of nickel (Ni), its electron configuration includes both the 3d and 4s orbitals. The 3d orbital can hold up to 10 electrons, while the 4s orbital can hold up to 2 .
To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). We first need to find the number of electrons for the Ni .
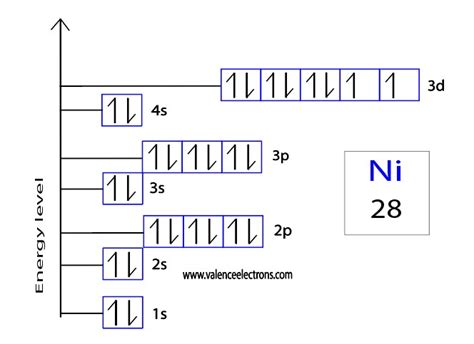
The nickel consists of 28 electrons in the atomic shell in 4 orbits. Electron configuration is the distribution of electrons in the orbits of atoms or molecules. The electronic configuration of nickel is: 1s 2 2s 2 . The orbital diagram of nickel shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 .
The p orbitals are px, py, and pz, and if represented on the 2p energy with full orbitals would look like: 2p x 2 2p y 2 2p z 2. The expanded notation for neon (Ne, Z=10) is written as follows: 1s 2 2s 2 2p x 2 2p y 2 2p z 2. The .The nickel orbital diagram can be represented using the following notation: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8 The notation indicates the distribution of electrons in each energy .Density: 8.91 g/cm 3 . Electronic configuration of the Nickel atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8Electronic configuration of the Nickel .
Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Nickel's atomic number is 28, so we need to arrange 28 electrons. Since this element has quite a high atomic number, it's faster if, instead of. See full answer below.
The d-orbital is cloverleaf-shaped and can hold a maximum of 10 electrons. Finally, the f-orbital is complex in shape and can hold a maximum of 14 electrons. In the case of nickel (Ni), its electron configuration includes both the 3d and 4s orbitals. The 3d orbital can hold up to 10 electrons, while the 4s orbital can hold up to 2 electrons.electron orbital notation worksheetDraw the ground state electron configuration using orbital notation for the following elements: P, Al, F. Draw representation of ground-state electron configuration using the orbital notation for Gallium. Draw the orbital diagram for an atom with an electron configuration of 1s22s22p63s23p3. Here’s how you can draw the orbital diagram of nickel step by step. #1 Find electrons of nickel. #2 Write electron configuration of nickel. #3 Draw orbital diagram of nickel. Let’s break down each step in detail.That is, the orbital notation of gold is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 1 4f 14 5d 10. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when multiple orbitals of the same energy level are available.
Which of the following is the correct orbital notation for the 3d sublevel of the element nickel, (Ni, atomic #28). ? ? ? ? The orbital notation depicted here is for the element: Check Hint Show answer. The "up" and "down" arrows in electron orbital notation, such as is shown here, depict: ? electrons and protons attracting each other .The carbon orbital notation is a shorthand system designed to represent the exact positions of the electrons in the carbon atom. This is similar to electron configuration, but numbers are used instead of boxes to represent the positions of the electrons. This orbital notation system always follows the Aufbau principle.

Located in the fourth period on the table of elements, nickel is a transition metal sandwiched between cobalt and copper. As each element adds an electron to one of the orbits, nickel adds its additional electron to the third orbital. It creates the electron configuration of 2-8-16-2. Industry finds nickel useful because it is malleable and can . Atomic Number of Nickel (Ni) = 28 Electronic configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d8 or [Ar], 3d8, 4s2 where [Ar] is Argon with atomic number 18. Answer link. Related questions. How do electron configurations in the same group compare? The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements!From Sc on, the 3d orbitals are actually lower in .In the case of nickel, the noble gas notation starts with the electron configuration of argon (1s^2 2s^2 2p^6 3s^2 3p^6), followed by the additional electron configuration of nickel (3d^8 4s^2). In terms of the orbital diagram, the 28 electrons in a nickel atom are distributed among the different orbitals. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. . Nickel (Ni) 29: Copper (Cu) 30: Zinc (Zn) 31: Gallium (Ga) 32: Germanium (Ge) 33: Arsenic (As) 34: Selenium (Se) 35: Bromine (Br) 36: Krypton (Kr) 37 .
Nickel # Ni_28 # has 28 electrons. 18 electrons fill up the third electron shell leaving 10 valance electrons. 2 electrons in the 4s and 8 elections in the 3d. When Nickel becomes #Ni^+2# Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.. The 4s electrons a lower energy level that the 3d electrons because of the .That is, the orbital notation of niobium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1 4d 4. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when multiple orbitals of the same energy level are available.That is, the orbital notation of hassium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 6. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when multiple orbitals of the same energy level are available.
The electronic configuration is defined as the number of electrons distributed in the atom’s or molecule’s orbits. Nickel has 28 electrons which are distributed among the 4 orbits of its atom. Electronic configuration of nickel can be written as: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2 or it can also be written as [Ar] 3d 8 4s 2.
The electron configuration for phosphorus is 1s 2 2s 2 2p6 3 s2 3p3 and the orbital diagram is drawn below. 1.4: Electron Configurations and Electronic Orbital Diagrams (Review) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom indicates the number of valence .Nickel is a d-block element having atomic number 28. The chemical symbol of Nickel is ‘Ni’ Electronic configuration of d-block. In general, the electronic configuration of these elements is (n-1) d 1-10 ns 1-2. Here, (n–1) stands for the inner d orbitals which may have one to ten electrons, and the outermost n s orbital may have one or .
Pacific Time. Pacific Time (PT) is a general term used to describe the areas that observe either the Pacific Standard Time (PST) or Pacific Daylight Time (PDT) in the United States and Canada. PT also called Pacific Coast Time. PT is not static but switches between PDT and PST. Territories observing the Pacific Time Zone are primarily in North America.
orbital notation for nickel|electron orbital notation worksheet